









INDICATIONS FOR USE:
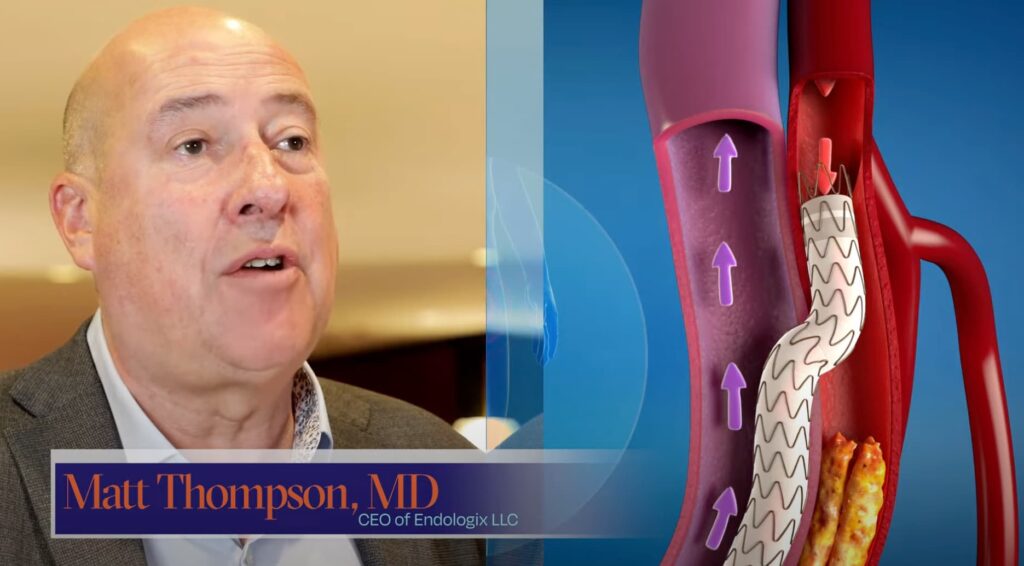
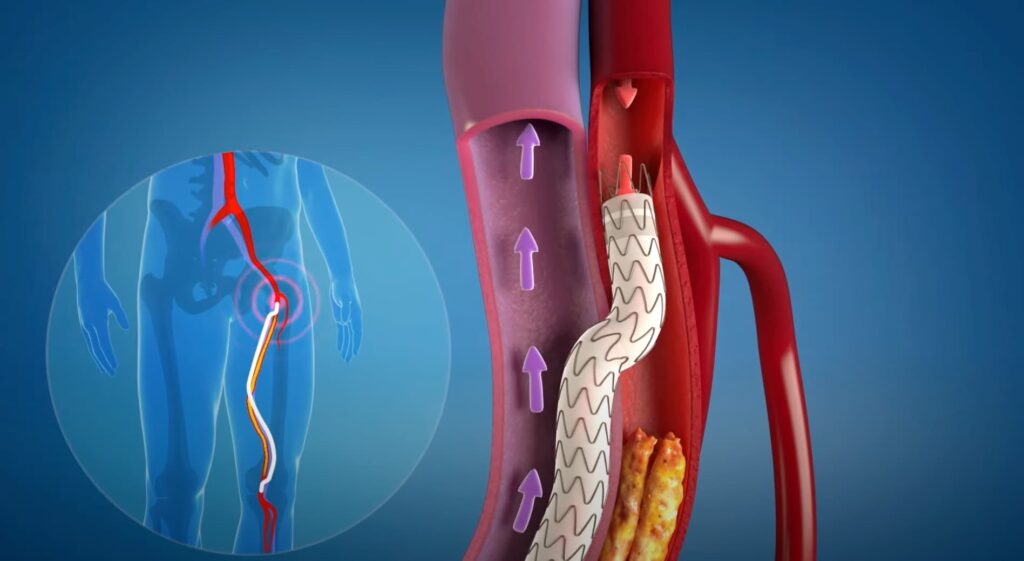
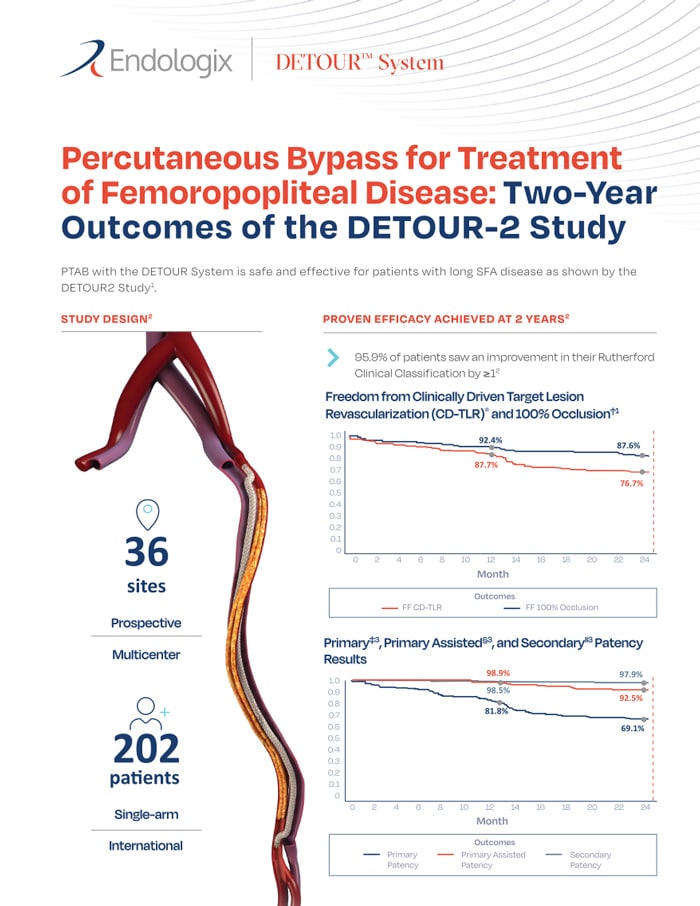
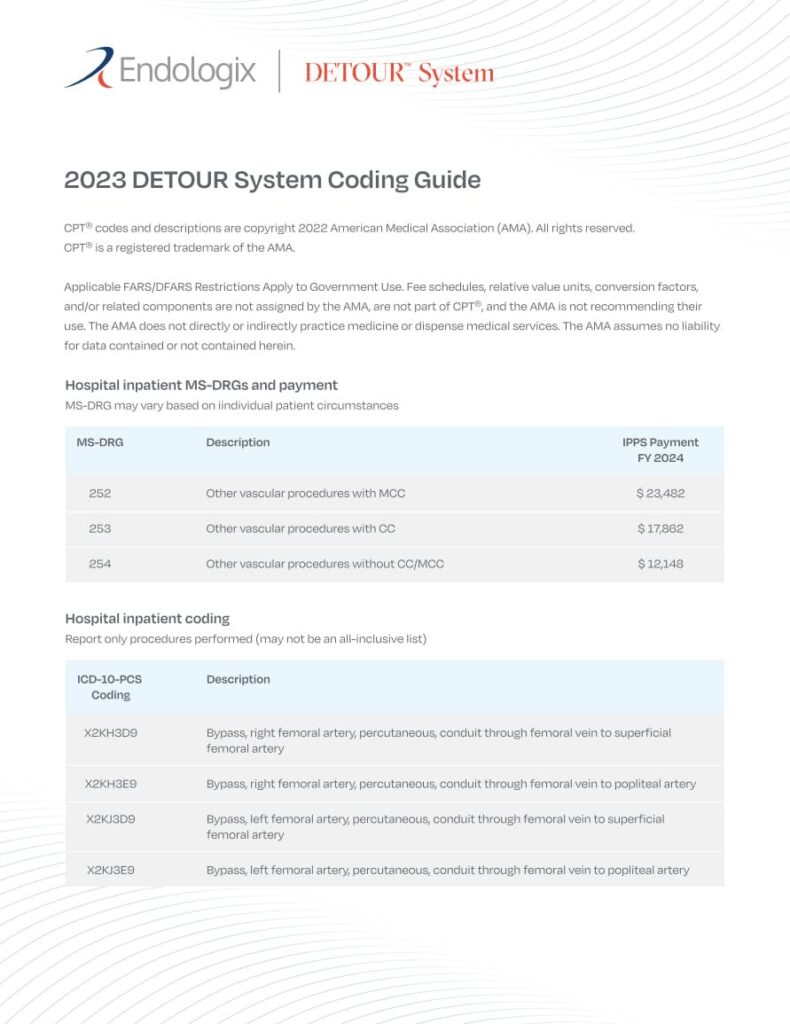
The DETOUR™ System is indicated for use for percutaneous revascularization in patients with symptomatic femoropopliteal lesions from 200mm to 460mm in length with chronic total occlusions (100mm to 425mm) or diffuse stenosis >70% who may be considered suboptimal candidates for surgical or alternative endovascular treatments. The DETOUR™ System, or any of its components, is not for use in the coronary and cerebral vasculature.
CONTRAINDICATIONS:
The DETOUR™ System is contraindicated in patients with:
Refer to Instructions for Use for more information concerning Indications, Contraindications, Specific Anatomic Considerations, Warnings, Precautions, and Adverse Events.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
NOTE: Not all product components are available in every country.
Please consult with your Endologix representative to confirm product availability.
Endologix® is a registered trademark of Endologix LLC in the United States, Europe and Japan. All other trademarks are the property of their respective owners.
©2023 Endologix LLC. All rights reserved. MM2741-US Rev 01