Explore our EVAR
portfolio resources.
“The Role of Adaptive Sealing Technology in Clinical Practice”

“The Role of Adaptive Sealing Technology in Clinical Practice” – featured in Endovascular Today
ENCORE (EffectiveNess of Custom Seal with Ovation: Review of the Evidence)
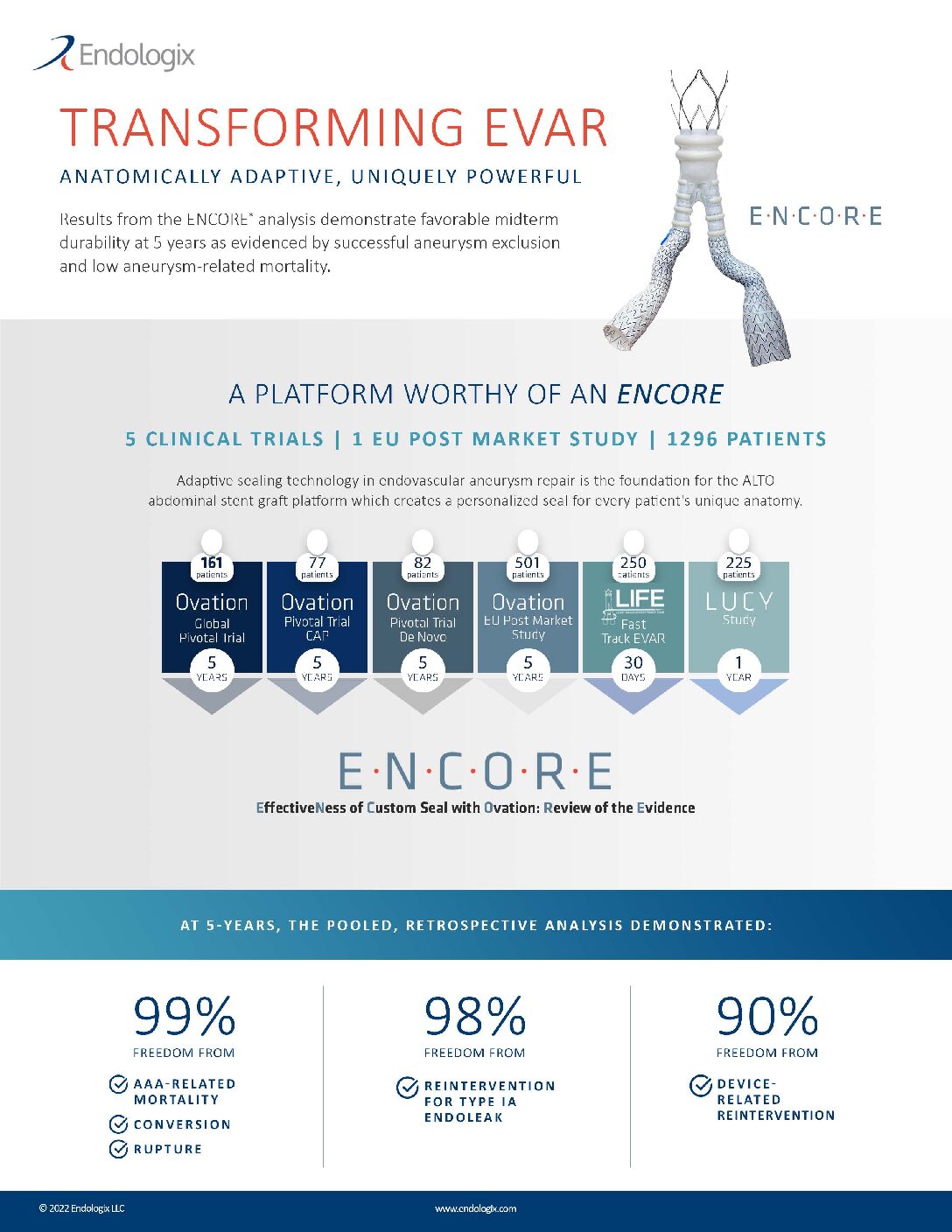
ENCORE (EffectiveNess of Custom Seal with Ovation: Review of the Evidence) Transforming
Evolving EVAR – The durability of adaptive sealing technology in specialized anatomies

Evolving EVAR–The durability of adaptive sealing technology in specialized anatomies
LEOPARD 5Yr Infographic

VQI – BMJ Infographic
ALTO Clinical Update

AFX2 Clinical Update

ALTO Product Brochure
ALTO Quick Reference Guide

ALTO Stent Graft System Overview

AFX2 Brochure

AFX2 Quick Reference Guide

AFX2 Stent Graft Selection Guide

National Webinar - The Role of Adaptive Sealing Technology in Clinical Practice

National Webinar - The Role of Adaptive Sealing Technology in Clinical Practice
ALTO Case Review with Dr. Beaulieu

ALTO Case Review with Dr. Mouawad

ALTO Case Review (1) with Dr. Ramaiah

ALTO Case Review (2) with Dr. Ramaiah

ALTO Case Review (3) with Dr. Ramaiah

AFX2 Webinar with Dr. Zach Arthurs – EVAR is Here to Stay

AFX2 Pre/Post Case Images

EVAR Solutions using ALTO - Drs. Justin George and Jarrad Rowse Webinar
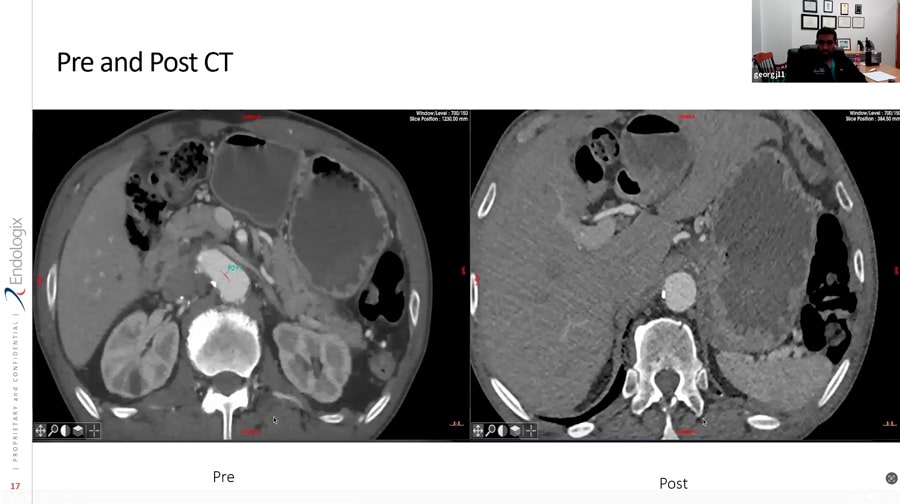
ALTO Roundtable Webinar with Drs. Mark Conrad, Anthony Feghali, and Luis Gomez
ALTO Roundtable Webinar with Drs. Mark Conrad, Anthony Feghali, and Luis Gomez
Dr. Luis Gomez ALTO Case Review
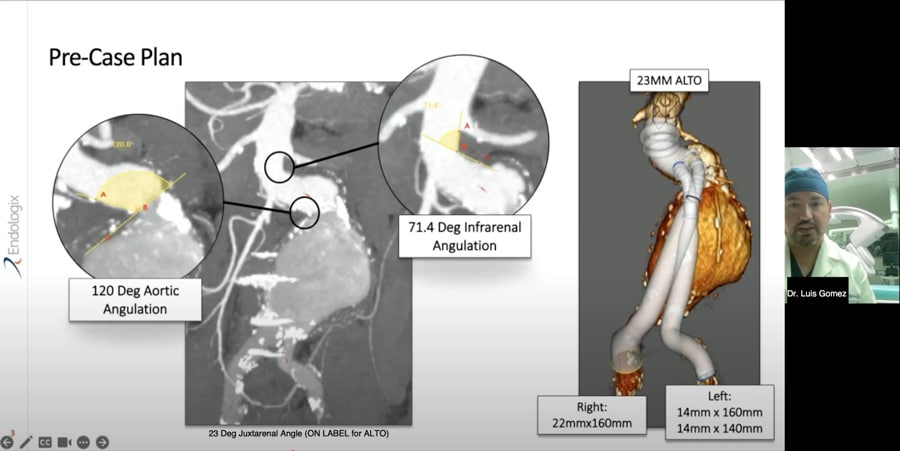
ALTO - Drs Justin George and Jarrad Rowse

EVAR Solutions using ALTO - Drs. Justin George and Jarrad Rowse Webinar
Physician AAA Coding Guide
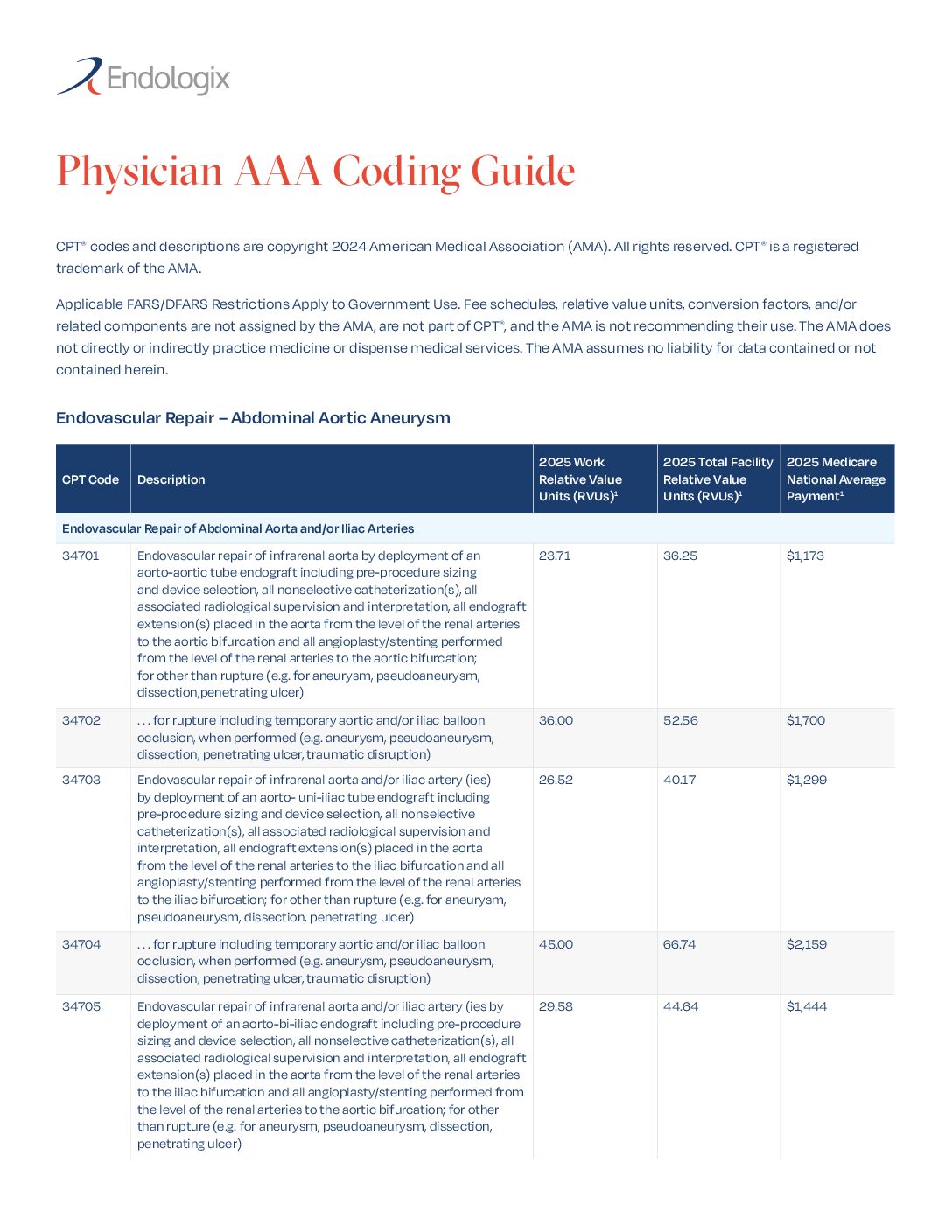
AAA Facility Coding & Reimbursement

AFX®2 Endovascular AAA System
INDICATIONS FOR USE – US:
The Endologix® AFX®2 Endovascular AAA Systems are indicated for treatment of patients with abdominal aortic aneurysms having the vascular morphology suitable for endovascular repair using a surgical vascular access technique or a bilateral percutaneous technique; a non-aneurysmal aortic neck between the renal arteries and the aneurysm: with length of ≥15mm, diameter ≥18 to ≤32mm and neck angle of ≤60° to the body of the aneurysm; aortic length ≥1.0cm longer than the body portion of the chosen bifurcated model; common iliac artery distal fixation site with length ≥15mm, diameter of ≥10 to ≤23mm, and with ability to preserve at least one hypogastric artery; and with an iliac angle of ≤90° to the aortic bifurcation. Extension stent grafts must have the ability to overlap the bifurcated stent graft by at least 30 to 40mm proximally and at least 15 to 20mm distally.
CONTRAINDICATIONS – US:
The Endologix® AFX®2 Endovascular AAA Systems are contraindicated for use in patents who have a condition that threatens to infect the graft and in patients with sensitivities or allergies to the device materials. Refer to the Instructions for Use for more information concerning Indications, Contraindications, Warnings and Precautions, and Adverse Events.
INDICATIONS FOR USE – EU:
The Endologix® AFX®2 Endovascular AAA Systems are indicated for treatment of patients with abdominal aortic aneurysms having the vascular morphology suitable for endovascular repair using a surgical vascular access technique; a non-aneurysmal aortic neck between the renal arteries and the aneurysm: with length of ≥15mm, diameter ≥18 to ≤32mm and neck angle of ≤60 to the body of the aneurysm; aortic length ≥1.0cm longer than the body portion of the chosen bifurcated model; common iliac artery distal fixation site with length ≥15mm, diameter of ≥10 to ≤ 23mm, and with ability to preserve at least one hypogastric artery; and with an iliac angle of ≤90° to the aortic bifurcation. Extension stent grafts must have the ability to overlap the bifurcated stent graft by at least 30 to 40mm proximally and at least 15 to 20mm distally.
CONTRAINDICATIONS – EU:
The Endologix® AFX®2 Endovascular AAA Systems are contraindicated for use in patents who have a condition that threatens to infect the graft and in patients with known sensitivities or allergies to the device materials. Refer to the Instructions for Use for more information concerning Indications, Contraindications, Warnings and Precautions, and Adverse Events.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. Rx only.
NOTE: Endologix products and associated components are not available in all countries or regions.
Please consult with your Endologix representative for details regarding product availability. CE marked.
Please refer to current product instructions for use.
ALTO® Abdominal Stent Graft System
INDICATIONS FOR USE:
The ALTO® Abdominal Stent Graft System is indicated for treatment of patients with infrarenal abdominal aortic aneurysms having the vascular morphology suitable for endovascular repair with the device, which includes the following:
- Adequate iliac/femoral access compatible with vascular access techniques (femoral cutdown or percutaneous), devices, and/or accessories,
- A proximal aortic landing zone for the sealing ring 7 mm below the inferior renal artery.
- An aortic sealing zone comprised of healthy aorta defined as:
- Lack of significant thrombus > 8 mm in thickness; at any point along the aortic circumference at the level of 7mm below the inferior renal artery, Lack of significant calcification at the level of 7 mm below the inferior renal artery,
- Conicity < 10% as measured from the inferior renal artery to the aorta 7 mm below the inferior renal artery,
- An inner wall diameter of no less than 16 mm and no greater than 30 mm at 7 mm below the inferior renal artery, and
- An aortic angle of ≤ 60 degrees
- A distal iliac landing zone:
- With a length of at least 10 mm, and
- With an inner wall diameter of no less than 8 mm and no greater than 25 mm.
CONTRAINDICATIONS
The system is contraindicated for use in patients who have a condition that threatens to infect the graft and in patients with known sensitivities or allergies to the device materials including polytetrafluoroethylene [PTFE], polyethylene glycol [PEG]-based polymers, contrast agents, fluorinated ethylene propylene [FEP], titanium, nickel, platinum, or iridium.
Refer to Instructions for Use for more information concerning Indications, Contraindications, Specific Anatomic Considerations, Warnings, Precautions, and Adverse Events.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
NOTE: Not all product components are available in every country.
Please consult with your Endologix representative to confirm product availability.
Endologix®, AFX®2, ALTO®, DuraPly®, VELA®, and ActiveSeal® are registered trademarks of Endologix LLC in the United States and certain foreign countries. All other trademarks are the property of their respective owners.
©2026 Endologix LLC. All rights reserved. MM2807-ALL Rev 01