Five-Year Results of the LEOPARD RCT now published in JVS.

AFX2 Endovascular AAA System
demonstrated positive 5-year results
and similar outcomes to commercially available endografts in the LEOPARD randomized controlled trial.
The LEOPARD trial is the first RCT comparing an anatomically fixated endograft (AFX/AFX2) to proximal fixation endografts; and demonstrated1 the following:
Freedom from rupture
Freedom
from ARM*
AFX/AFX2 type III endoleak rate at 5 years
“Importantly, the comparable performance results from the LEOPARD study between AFX/AFX2 and other endografts align with a recent publication1 that was authored on behalf of the Society for Vascular Surgery’s Patient Safety Organization and used linked registry claims data”
Matt Thompson, MD

^Publication Summary from BMJ: Use of linked registry claims data for long term surveillance of devices after endovascular abdominal aortic aneurysm repair: observational surveillance study2
The published study set out to evaluate long term outcomes (reintervention and late rupture of abdominal aortic aneurysm) of aortic endografts in real world practice using linked registry claims data.
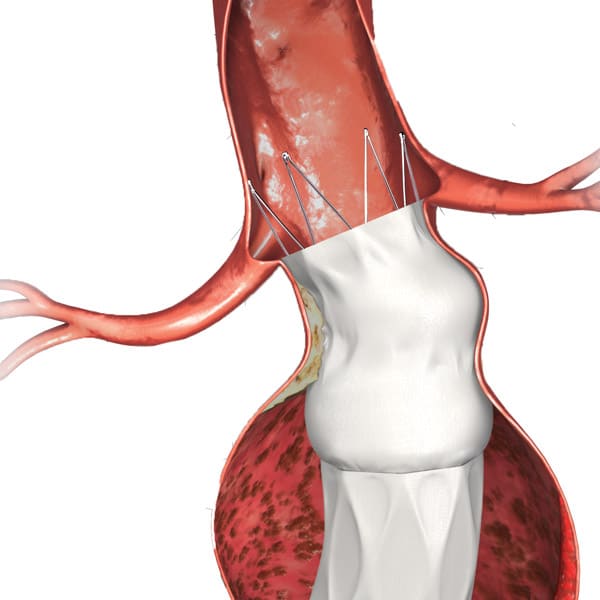
Overcome common challenges seen in specific anatomies.
Bifurcated unibody design preserves the native bifurcation and separates graft fixation from the sealing zone
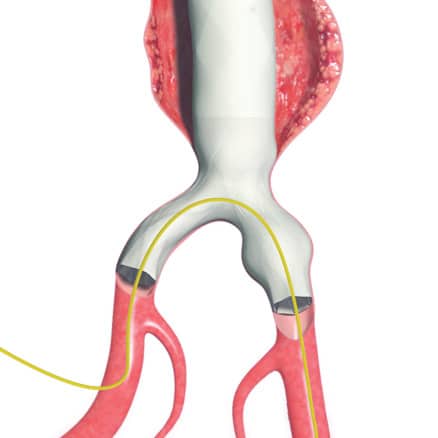
AFX2 System’s ActiveSeal® technology conforms to the aortic wall
Less Time, Intuitive Design, Greater Efficiency
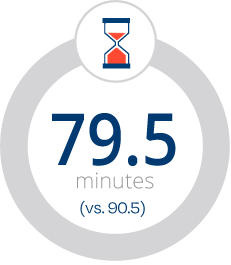
Shorter procedure times

Reduced fluoroscopy time
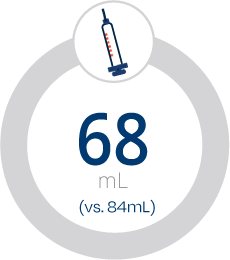
Less contrast volume
Explore how you can save time and resources
with the AFX2 System
Contact Us:
I want to explore further:
For information on Endologix’s privacy practices, see our Privacy Statement
- Kwolek CJ, Ouriel K, Stucky FS, et al. Five-year results of the Leopard Trial of commercially available endografts. Journal of Vascular Surgery. Published online 2023. doi:10.1016/j.jvs.2023.04.011
- Goodney, P, Mao J, Columb J, Suckow B, Schermerhorn M, Malas M, et al. Use of linked registry claims data for long term surveillance of devices after endovascular abdominal aortic aneurysm repair: observational surveillance study. BMJ 2022;379:e071452.
AFX2 Endovascular AAA System
INDICATIONS FOR USE (US):
The Endologix® AFX®2 Endovascular AAA System is indicated for endovascular treatment in patients with AAA using a surgical vascular access technique or a bilateral percutaneous technique. The devices are indicated for patients with suitable aneurysm morphology for endovascular repair, including: Adequate iliac/femoral access compatible with the required delivery systems (diameter ≥ 6.5 mm); Non-aneurysmal aortic neck between the renal arteries and the aneurysm: with a length of ≥ 15 mm; with a diameter of ≥ 18 mm and ≤ 32 mm; with a neck angle of ≤ 60° to the body of the aneurysm. Aortic length ≥ 1.0 cm longer than the body portion of the chosen bifurcated model. Common iliac artery distal fixation site: with a distal fixation length of ≥ 15 mm; with ability to preserve at least one hypogastric artery; with a diameter of ≥ 10 mm and ≤ 23 mm; with an iliac angle of ≤ 90° to the aortic bifurcation. Extension stent grafts must have the ability to overlap the bifurcated stent graft by at least 30 to 40 mm proximally and at least 15 to 20 mm distally.
INDICATIONS FOR USE (EU):
The Endologix® AFX®2 Endovascular AAA System is indicated for endovascular treatment in patients with AAA using a surgical vascular access technique. The devices are indicated for patients with suitable aneurysm morphology for endovascular repair, including: Adequate iliac/femoral access compatible with the required delivery systems (diameter ≥ 6.5 mm); Non-aneurysmal aortic neck between the renal arteries and the aneurysm: with a length of ≥ 15 mm; with a diameter of ≥ 18 mm and ≤ 32 mm; with a neck angle of ≤ 60° to the body of the aneurysm. Aortic length ≥ 1.0 cm longer than the body portion of the chosen bifurcated model. Common iliac artery distal fixation site: with a distal fixation length of ≥ 15 mm; with ability to preserve at least one hypogastric artery; with a diameter of ≥ 10 mm and ≤ 23 mm; with an iliac angle of ≤ 90° to the aortic bifurcation. Extension stent grafts must have the ability to overlap the bifurcated stent graft by at least 30 to 40 mm proximally and at least 15 to 20 mm distally.
CONTRAINDICATIONS:
The Endologix® AFX®2 Endovascular AAA System is contraindicated for use in patients who have a condition that threatens to infect the graft and in patients with sensitivities or allergies to the device materials. Refer to the Instructions for Use for more information concerning Indications, Contraindications, Warnings and Precautions, and Adverse Events.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. Rx only.
Note: Endologix products and associated components are not available in all countries or regions. Please consult with your Endologix representative for details regarding product availability.
CE marked. Please refer to current product instructions for use.
Endologix®, AFX®2, ALTO®, DuraPly®, VELA®, and ActiveSeal® are registered trademarks of Endologix LLC in the United States and certain foreign countries. All other trademarks are the property of their respective owners.
©2023 Endologix LLC. All rights reserved. MM2635-ALL Rev 01