Proven to Meet the Needs of Female EVAR Patients
Improving Outcomes with an Exceptional EVAR Stent Graft
Unique challenges face females in healthcare, including in AAA repair. Historically, females have been underserved by conventional EVAR. Only 34% of females with AAA (v. 54% of males) are eligible for EVAR treatment due to anatomical restrictions. Females need an exceptional EVAR treatment to meet their complex anatomical needs.


For every woman. For life.
We remain focused.
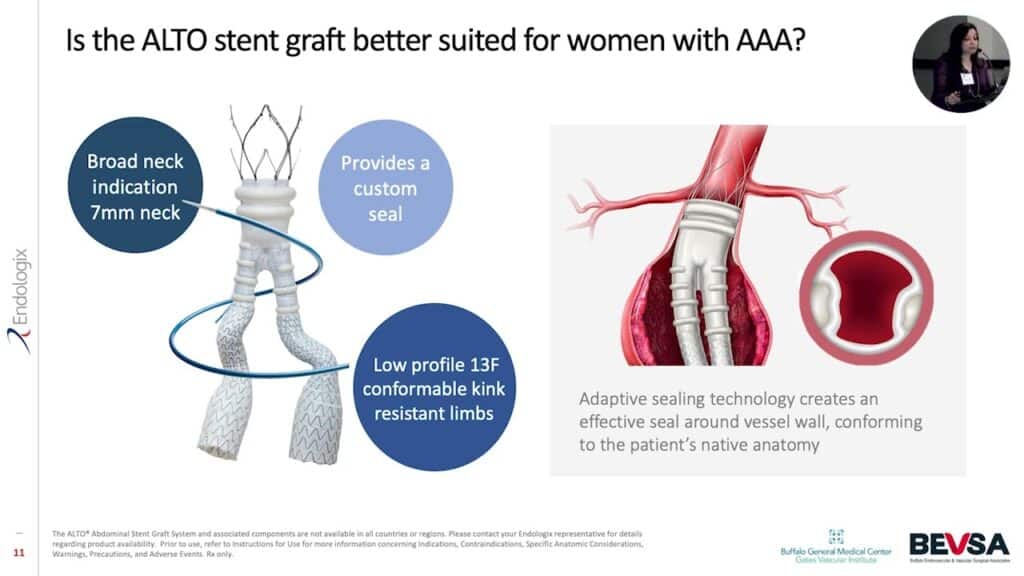
Alto Stent Graft
Endologix focused on and validated the Alto Stent Graft to meet the anatomical needs of females. Choose an EVAR treatment designed to meet the unique needs of the complex female anatomy.
Challenge
Solution
Excessive Aortic Neck Angulation

Short Proximal Neck Length
Separation of fixation and seal allows you to fixate graft in healthy tissue of the aorta and seal closest to renals4
Smaller External Iliac Artery Diameter
Lowest profile device on the market – 13F inner diameter and 15F outer diameter5

Challenge
Extreme Aortic Neck Angulation
Solution
Exclusive adaptable sealing technology creates an effective seal around the vessel wall conforming to the patient’s native anatomy4

Challenge
Short Proximal Neck Length
Solution
Separation of fixation and seal allows you to fixate graft in healthy tissue of the aorta and seal closest to renals.

Challenge
Smaller External Iliac Artery Diamete
Solution
Lowest profile device available 13 F ID / 15F OD delivery system with integrated sheath.

Why Choose Endologix?
Endologix is committed to the steadfast pursuit of focused innovation, guided by data. Approach challenging anatomies with confidence and meet the unique needs of your complex patients.
We strive to ally with physicians for procedural success and proficiency, including:

Immersive procedural training with virtual reality (VR)
Comprehensive training programs for each product and therapy. Meet with your field team to create your training plan.


Dedicated in-procedure support from expert field team (in every case with you)
Strong Peer to peer network and support including: PTAB and EVAR communities, roundtables, and more

EVAR solution driven by data to meet the needs of complex female anatomy.
- Ulug P, Sweeting MJ, von Allmen RS, Thompson ST, Powell JT. Lancet 2017; 389(10089):2482-2491.
- Solberg et al. Eur J Vasc Endovasc Surg 2005; 29:145-149
- Analysis based on available data from the LUCY Study female cohort (72 out of 76) and on comparisons with grafts ranging from 18F – 21F OD manufactured by global market leaders. Data extracted on May 1, 2017. The Ovation Abdominal Stent Graft System has not been studied in a head-to-head clinical study against other EVAR devices for outcomes in women.
- Varkevisser et al. J Vasc Surg 2019
- V.N. Tedjawirja, M.C.J. de Wit, R. Balm, M.J.W. Koelemay, Annals of Vascular Surgery, 2021: 76: 330-341
INDICATIONS FOR USE:
The ALTO® Abdominal Stent Graft System is indicated for treatment of patients with infrarenal abdominal aortic aneurysms having the vascular morphology suitable
for endovascular repair with the device, which includes the following:
- Adequate iliac/femoral access compatible with vascular access techniques (femoral cutdown or percutaneous), devices, and/or accessories,
- A proximal aortic landing zone for the sealing ring 7 mm below the inferior renal artery.
- An aortic sealing zone comprised of healthy aorta defined as:
- Lack of significant thrombus > 8 mm in thickness; at any point along the aortic circumference at the level of 7mm below the inferior renal artery,
- Lack of significant calcification at the level of 7 mm below the inferior renal artery,
- Conicity < 10% as measured from the inferior renal artery to the aorta 7 mm below the inferior renal artery,
- An inner wall diameter of no less than 16 mm and no greater than 30 mm at 7 mm below the inferior renal artery, and
- An aortic angle of ≤ 60 degrees
- A distal iliac landing zone:
- With a length of at least 10 mm, and
- With an inner wall diameter of no less than 8 mm and no greater than 25 mm.
CONTRAINDICATIONS: The system is contraindicated for use in patients who have a condition that threatens to infect the graft and in patients with known sensitivities or allergies to the
device materials including polytetrafluoroethylene [PTFE], polyethylene glycol [PEG]-based polymers, contrast agents, fluorinated ethylene propylene [FEP], titanium, nickel, platinum, or iridium.
Refer to Instructions for Use for more information concerning Indications, Contraindications, Specific Anatomic Considerations, Warnings, Precautions, and Adverse Events.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
NOTE: Not all product components are available in every country. Please consult with your Endologix representative to confirm product availability.
Endologix is a registered trademark of Endologix LLC in the United States, Europe and Japan and ALTO is a registered trademark of Endologix LLC and its subsidiaries. All other trademarks are the property of their respective owners.
©2025 Endologix LLC. All rights reserved. MM2890-ALL Rev 01
Let’s Get Started
I would like to explore how I can treat my female patients with an exceptional stent graft