WHY PTAB with the DETOUR System?
Patients with long complex SFA disease face treatment tradeoffs, and some have no desirable options. Endovascular interventions can require multiple re-interventions, while open surgical bypass can pose significant risks, lengthy recovery times, and limited long‑term durability.
Endovascular Interventions
1 in 4
patients will need reintervention at one year3
Open Surgical Bypass
5.7
days in hospital
on average4
4.5 - 14%
infection rate
post surgery3,5-7
WHAT is the DETOUR System?
Introducing the First Fully Percutaneous Bypass Solution
PTAB with the DETOUR System is the first minimally invasive procedure that effectively bypasses long femoropopliteal lesions. PTAB with the DETOUR System is a clinically-proven minimally invasive therapy designed for PAD patients who have long femoropopliteal lesions, and those who may not good candidates for surgical bypass or repeat endovascular procedures.
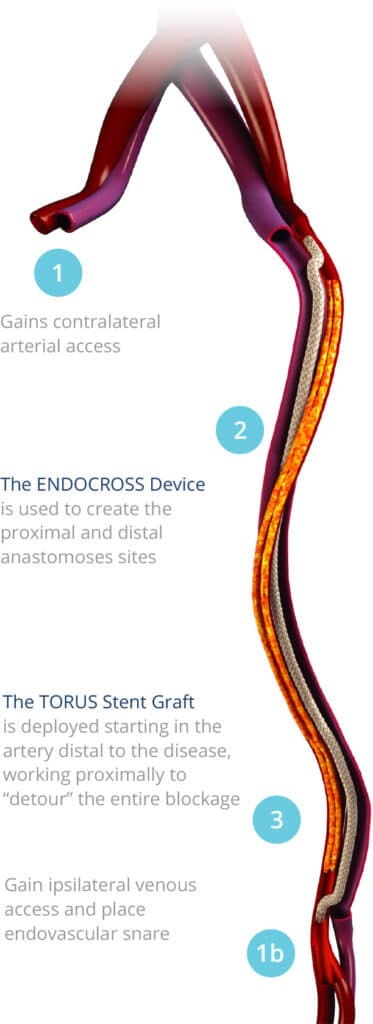
Under fluoroscopic guidance, the DETOUR System creates a femoropopliteal bypass routed through the femoral vein, delivering unobstructed flow from the superficial femoral artery (SFA) to the popliteal artery.
- ENDOCROSS™ Device: Creates precise arterial-venous connections
- TORUS™ Stent Graft: Engineered specifically for optimal blood flow and long-term durability. Available in multiple sizes (5.5-6.7mm diameter, 100-200mm length) and designed with radial strength for lasting results.
Clinical Evidence That Matters
Clinical studies continue to validate the safety and effectiveness of PTAB using the DETOUR System, particularly in long, complex SFA disease. The DETOUR2 Study demonstrates promising patency, low complication rates, and shorter hospital stays compared to other treatment options.
DEMONSTRATED EFFICACY
at 3 years2
DEMONSTRATED SAFETY
at 30 days2
30 days1
MINIMAL HOSPITAL STAYS
post-procedure2
Ready to bring this novel therapy to your practice?
Real World Patient Case Examples
Explore the types of patients that may benefit from PTAB with the DETOUR System, and patient cases that highlight the demographics, clinical presentation, and procedural outcomes.
- Long segment SFA lesions, recurrent CTO but not crossable, symptomatic claudicants with long lesions.
- Poor open surgical candidates, very co-morbid (CHF, COPD, ESRD)
- Recurrent occlusion, failed multiple endovascular treatments.
- Critical limb ischemia patients.
- Failed prior surgical bypass.
- Patients’ refusal of surgical bypass.
Hear From Patients Just Like Yours
Watch PTAB patients Mae Malcom and Victoria Liu-Stephen share their treatment journeys and success stories.
Contact Your Local Representative
Ready to learn more about bringing DETOUR to your practice? Our Endologix representatives are here to help you bring this novel therapy to your practice.
- Lyden. Percutaneous Bypass for Treatment of Long-Segment Femoropopliteal Disease: 12 Month Results from the DETOUR 2 Trial Volume 75, Issue 6, E337-E338, June 2022
- Lyden. Results of the DETOUR2 Study: Durability of Percutaneous Transmural Arterial Bypass for Treatment for Complex Femoropopliteal Disease. VAM 2024
- Kim TI, Zhang Y, Cardella JA, Guzman RJ, Ochoa Chaar CI. Outcomes of bypass and endovascular interventions for advanced femoropopliteal disease in patients with premature peripheral artery disease. J Vasc Surg. 2021 Dec;74(6):1968-1977.e3. doi: 10.1016/j.jvs.2021.05.034. Epub 2021 Jun 6. PMID: 34090986.
- Medicare open surgical bypass procedures based on 2021 data
- Kim TI, Zhang Y, Cardella JA, Guzman RJ, Ochoa Chaar CI. Outcomes of bypass and endovascular interventions for advanced femoropopliteal disease in patients with preShah, T, Tirziu, D, Ghare MI, Yang Y, Taoutel R, Gaston, S, Pietras C, Lansky AJ. Surgical Bypass of Femoral-Popliteal Arterial Disease: A Meta-analysis of Randomized and Prospective Trials. J CRIT LIMB ISCHEM 2022;2(4):E122-E130
- Voicu S, Trooboff SW, Goodney PP, Zwolak RM, Powell RJ. Medicare reimbursement of lower extremity bypass does not cover cost of care for most patients with critical limb ischemia. J Vasc Surg. 2020 Sep;72(3):1068-1074. doi: 10.1016/j.jvs.2020.01.062. PMID: 32829764
- Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FG, Gillepsie I, Ruckley CV, Raab G, Storkey H; BASIL trial participants. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005 Dec 3;366(9501):1925-34. doi: 10.1016/S0140-6736(05)67704-5. PMID: 16325694.
INDICATIONS FOR USE:
The DETOUR™ System is indicated for use for percutaneous revascularization in patients with symptomatic femoropopliteal lesions from 200mm to 460mm in length with chronic total occlusions (100mm to 425mm) or diffuse stenosis >70% who may be considered suboptimal candidates for surgical or alternative endovascular treatments. The DETOUR™ System, or any of its components, is not for use in the coronary and cerebral vasculature.
CONTRAINDICATIONS:
The DETOUR™ System is contraindicated in patients with:
- A distal common femoral artery (CFA) <7 mm in diameter.
- Increased risk of deep vein thrombosis (DVT), such as patients with a recent history of DVT, thrombophilia, and disseminated malignancy.
- Untreated flow-limiting aortoiliac occlusive disease.
- Lack of patent single vessel tibial runoff to ankle.
- Known coagulopathy, bleeding diathesis, or thrombocytopenia that cannot be medically managed.
- Known hypersensitivities, allergies or contraindications to: Nitinol; PTFE; aspirin; heparin; antiplatelet; anticoagulant or thrombolytic therapy; or contrast media that cannot otherwise be medically managed.
Refer to Instructions for Use for more information concerning Indications, Contraindications, Specific Anatomic Considerations, Warnings, Precautions, and Adverse Events.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
NOTE: Not all product components are available in every country.
Please consult with your Endologix representative to confirm product availability.
Endologix® is a registered trademark of Endologix LLC in the United States, Europe and Japan. All other trademarks are the property of their respective owners.
©2025 Endologix LLC. All rights reserved. MM2889-US Rev 01